In 2004 the Mercer Museum in Doylestown, Pennsylvania, brought this Vampire Killing Kit to Winterthur’s Scientific Research and Analysis Laboratory (SRAL) for authentication. Such a project was something new for us. Although the lab routinely undertakes analysis to identify materials used to make various objects, we were unsure whether our analysis would provide the information needed to authenticate the kit.
Prosecution
Although the materials used to make some of the components of this Vampire Killing Kit are consistent with a 19th-century date, there are some important anomalies. Perhaps the most significant is the presence of optical brighteners in the paper labels inside the lid and on the garlic box. Optical brightening agents were introduced into paper manufacture about 1945, so these labels cannot date to the 19th century.
The presence of barium in the optical glass in the hand-held magnifier indicates a fairly recent date. The “silver” bullets were found to be pewter.
A wide variety of Vampire Killing Kits have come on the market in recent years, and some people even advertise that they make them. Many have components with labels referencing a mysterious Dr. Ernst Blomberg, who despite extensive research has not been positively identified.
And, by the way, do you believe in vampires?
Defense
In the 19th century, the belief in vampires spread panic in parts of Europe and America. In 1854 several corpses in Jewett City, Connecticut, had been exhumed in the belief that they were vampires. Given this history, wouldn’t we expect that Vampire Killing Kits might well have been created in response?
Although some of the components have been found to be of a later date, they might just represent replacements and repairs to a complex original set of implements. The box itself is old, as is the gun that fits perfectly. Vampire Killing Kits have been sold through reputable auction houses and purchased by reputable museums. The gunpowder found in the powder horn was discovered to be genuine.
Scientific Analysis
The components of the kit were examined for consistency with the proposed date of 1830−60. Energy dispersive X-ray fluorescence analysis (XRF) was undertaken to determine the elemental composition of the various parts. Fourier-transform infrared spectroscopy (FTIR) was undertaken to identify the organic components. Some components were examined under both long- and short-wave ultraviolet light (UV).
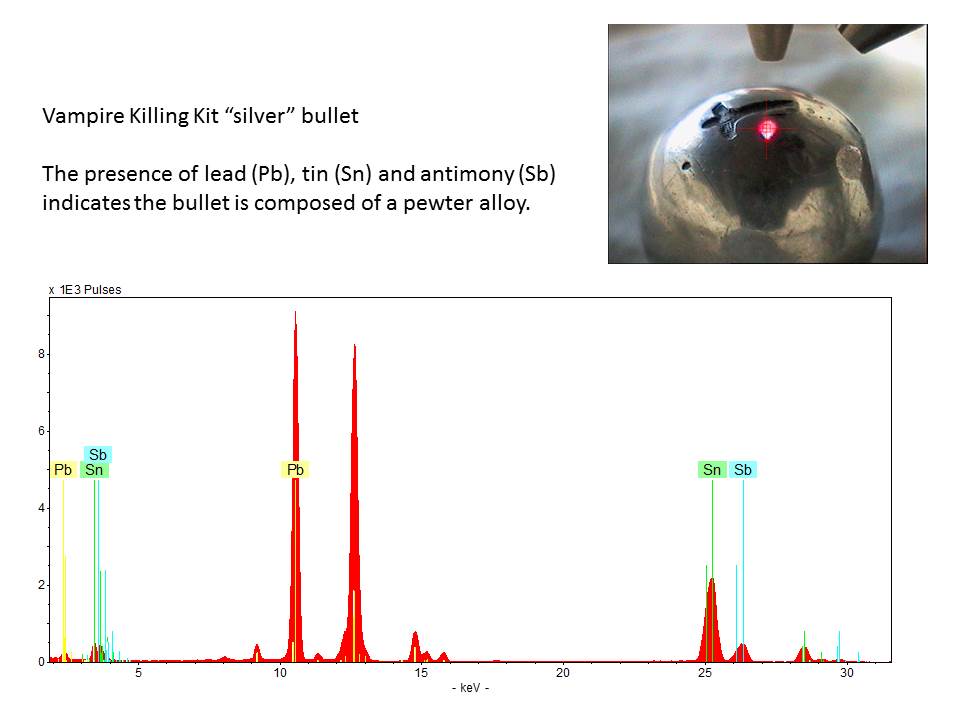
This photograph shows the “silver” bullet being analyzed using XRF. The detection of lead (Pb), tin (Sn), and antimony (Sb) indicates the bullet is composed of a pewter alloy. No silver was detected.
When exposed to ultraviolet light (UV), both the label inside the lid of the box and the ivory cross fluoresce. While this indicates that the label was made after 1945, the fluorescence of the cross indicates that it is really made of ivory and is probably a genuine antique.
Results:
- Gun barrel and breach. XRF: iron
- Bullet mold. XRF: iron, (manganese)
- Facing on the cross. XRF: calcium, FTIR: proteinaceous, good match for ivory. Blue-white fluorescence under long wavelength UV light also indicative of ivory.
- Facing nail on the cross. XRF: copper, zinc, nickel. Typical for German Silver
- Cross black paint/stain. XRF: No response, no mineral content in stain
- “Silver bullet.” XRF: lead, tin, antimony. Typical for pewter
- “Lead bullet.” XRF: lead.
- Magnifier brass. XRF: copper, zinc, trace iron. Typical for brass
- Magnifier glass. XRF: barium, bromine, potassium, arsenic, antimony. The presence of barium and antimony in apparently high concentration suggests a more modern optical glass.
- Mother-of-pearl inlay. XRF: calcium, strontium. Consistent for mother-of-pearl.
- Powder horn cap. XRF: copper, zinc (brass).
- Red capped vial, glass. XRF: low response, calcium, lead, iron.
- Red capped vial, serum cap. XRF low response, zinc, calcium, strontium, arsenic, lead, iron.
- Syringe barrel. XRF: lead, calcium. lead glass. Strong fluorescence under short wavelength UV light characteristic of high lead glass.
- Syringe plunger. XRF: lead, calcium. lead glass. Strong fluorescence under short wavelength UV light characteristic of high lead glass.
- Syringe cap. XRF: zinc, calcium. FTIR: Good match with hemicellulose. Microscopic examination and comparison with authentic sample suggests “vegetable ivory” or “rain forest ivory,” made from the tagua nut as an ivory substitute in the nineteenth century.
- Syringe needle. XRF: silver, copper (no gold or lead). Highly refined silver, post 1850
- Adhesive around the mother-of-pearl inlay. FTIR: Good match with polyvinyl acetate. Could be a repair with a modern adhesive?
- Leather case sample. FTIR: Good matches with protein material like leather.
- Green felt fiber. FTIR: Good match with wool.
- Adhesive on felt dividers. FTIR: Possible mixture of resin and animal glue.
- Coating on wooden dividers. FTIR: Shellac. Orange fluorescence under long wavelength UV light.
- Paper labels on box of garlic and inside lid of vampire kit. Blue-white fluorescence under long wavelength UV light shows the presence of optical brighteners in the paper. Optical brightening agents in paper are a post 1945 development.
- Powder horn. Contains gunpowder!
What is your verdict? Genuine or fake?
Bibliography
Petersen, W. C. Analytical Report # 4641, September 29, 2004 (unpublished report, Winterthur).
Video about the Vampire Killing Kit can be seen on the Travel Channel website: http://www.travelchannel.com/shows/mysteries-at-the-museum/video/vampire-killing-kit